

Multimodal Deep Learning Model for cancer prediction
Pathologists who specialize in studying histopathological tumor images play a crucial role in personalized cancer treatment. By examining a tumor tissue samples, they analyze the structure, composition, and behavior of cancer cells. This process helps identify the specific type of tumor, its grade, and its potential aggressiveness. Their work goes beyond basic diagnosis, as they also identify molecular and genetic markers that provide insights into how a particular tumor may respond to different treatments. These insights allow oncologists to tailor therapies to the individual characteristics of a patient’s cancer, leading to more effective and targeted treatment plans. This personalized approach helps improve patient outcomes, reduces unnecessary side effects, and ensures that treatments are more aligned with the biological profile of the tumor. The precision and expertise of pathologists are invaluable in the evolving field of precision medicine, where cancer treatments are increasingly based on a deep understanding of the tumor's unique biology.
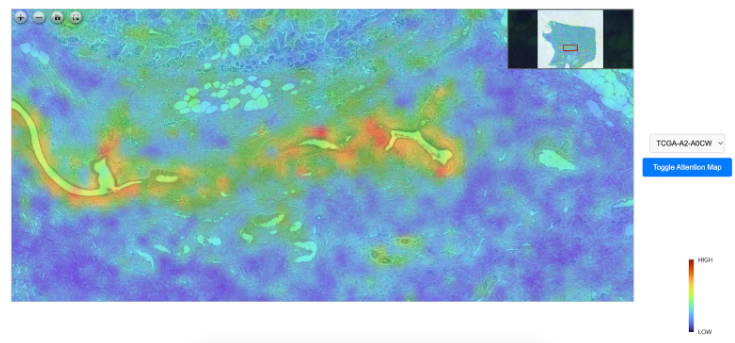
While pathologists play a pivotal role in diagnosing cancer and guiding personalized treatments, their analyses are often limited in scope. Traditionally, they rely primarily on examining histopathological slide images, which provide crucial insights into the tumor’s cellular structure and behavior. However, this approach only captures a part of the bigger picture. The cellular morphology seen on slides may not reveal the full complexity of the tumor's biology, particularly at the molecular or genetic level. Pathologists may often not have access to or fully integrate other critical data, such as the patient’s genomic profile or broader clinical information. Genomic data, for instance, can uncover specific mutations, biomarkers, or pathways driving cancer, which are not visible in tissue slides but are essential for identifying the most effective targeted therapies. Without incorporating these advanced molecular insights, the treatment recommendations may lack the full precision that modern cancer care demands.
NaCAGAT offers a promising solution to bridge the gap between traditional histopathological analysis and genomic data, providing pathologists with a powerful tool for more comprehensive cancer assessments. By integrating both histopathological slide images and genomic information, these models can analyze and correlate the visual characteristics of tumors with their underlying genetic drivers. This combination enables a deeper understanding of the tumor’s biology, offering insights that neither modality could provide on its own.
For pathologists, this AI-powered approach acts as an invaluable assistant, augmenting their expertise by revealing patterns and connections that may otherwise be missed. By synthesizing data from multiple sources, multimodal deep learning can guide more precise diagnoses, predict treatment responses, and support the development of highly personalized treatment plans. Ultimately, this technology enhances the pathologist’s ability to make data-driven decisions, paving the way for more targeted and effective cancer therapies tailored to the individual patient’s unique tumor profile.
